Professor Dengsong Zhang
5th November 2024
In this blog series, which is part of our Sustainability Hub, we’re speaking to chemical engineers across the world making a difference to make sustainable practices and products a reality and more accessible to all for the wider benefit of our society and globe.

Professor Dengsong Zhang is a distinguished scholar at Shanighai University with extremely high creativity in the field of environmental chemistry, in particular on energy and environmental catalysis.
He has boasts nearly 15 years’ expertise in catalytically transform greenhouse gas, air pollutants, and water pollutants to clean products in complicated working conditions via developing efficient, safe, and cheap catalytic technologies.
He held over 70 China and foreign patents and his research activity is internationally recognized in more than 270 scientific journal papers to date. He has a citation of over 24,090 with an h-index of 92.
Professor Zhang, tell us about your work in water treatment
In the early stages, my team and I concentrated on seawater and brackish water desalination technologies and obtained significant achievements in coupling ultrafiltration membranes and nano-filtration membranes with capacitive deionization (CDI), which enabled us to effectively remove salts and other impurities, achieving highly-efficient desalination of seawater and brackish water.
Our efforts led to the development of a series of desalination devices designed to address the scarcity of drinking water (Figure 1). These devices are user-friendly and adaptable to various environments, providing conveniently-treated and clean drinking water to residents. One notable achievement was the successful establishment of a demonstration project for seawater desalination on a small island in Zhejiang Province, supplying fresh water to local residents.
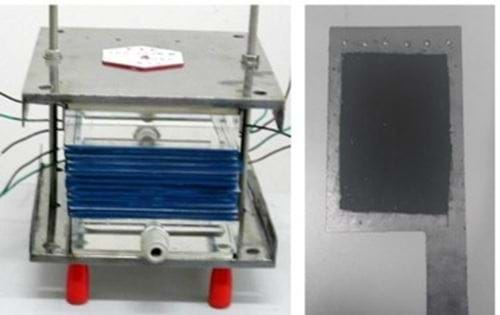
We have also actively promoted the application of brackish water desalination in new scenarios, securing supports from various high-profile projects, including national-level projects, Shanghai municipal projects, and industrial projects.
Our collaborations with companies such as Shanghai Construction Group Corporation and Shanghai Urban Construction Group Corporation underscore the practical values of our work.
Understanding that high energy consumption and costs have been major barriers against the widespread application of seawater desalination, we have developed high-performance seawater desalination electrodes to improve existing technologies, which significantly enhanced energy utilization efficiency and reduced costs with comparative energy of 0.32 Wh/g NaCl . For example, by modifying the surface hydrophilicity and pore structure of carbon materials, the CDI capacity of resulted carbon materials was highly increased. This research not only facilitates the commercialization of seawater desalination technology but also enhances its potential application in wastewater treatment, contributing significantly to environmental protection.
Moreover, our team is committed to education and talent cultivation, having trained many outstanding researchers and engineers for enterprises and universities. This educational focus ensures a continuous influx of talent, injecting new vitality and momentum into the future development of the water treatment field.
Tell us how you have been able to reduce energy consumption and carbon emissions through your work?
Achieving zero emissions is a global target and a crucial challenge for sustainable development of human society. Although capacitive deionization (CDI) is a relatively low-energy process, the improvement of deionization efficiency remains a priority.
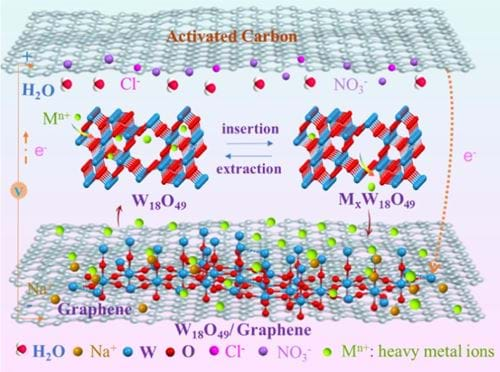
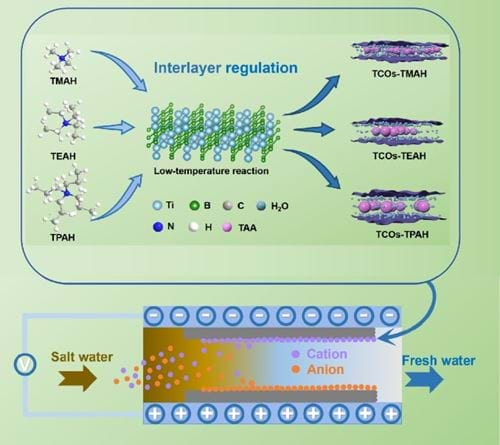
In our research on seawater desalination, we have successfully reduced energy consumption by designing electrode materials that enhance electrosorption capacity and improve the deionization efficiency. Our team also exert our efforts on applying the CDI technology to wastewater treatment, targeting the selective removal of harmful elements such as fluoride, nitrate, phosphorus, and heavy metals (Figure 2). It’s still very difficult to selectively remove these ions by using traditional carbon materials, so we have developed new faradaic electrode materials (Figure 3) to achieve selective ion removal via the mechanism of ion exchange and ion intercalation.
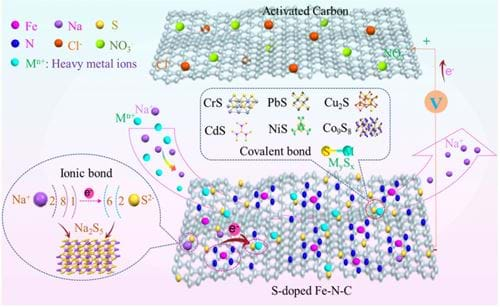
We are also trying to install industrialized device for the selective removal of harmful elements in wastewater systems based on these exciting lab-scale results.
Although faraday materials could improve capacitance and achieve selective removal, most Faraday materials have poor electrical conductivity, and the structure is prone to collapse after ion intercalation and deintercalation.
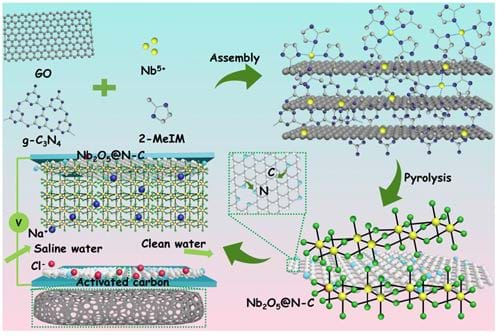
What we did was combining Faradaic materials with carbon materials (Figure 4) and introducing encapsulating agents into two-dimensional layered materials (Figure 5).
These approaches successfully improved conductivity, stabilized structures and enhanced cycle stability. Additionally, we explored the coupling of CDI with electro-Fenton technology, using hydroxyl radicals produced by electro-Fenton to oxidize and break heavy metal complexes, and then using CDI to capture heavy metal ions in situ, thus efficiently removing heavy metal complexes.
We have also successfully applied CDI technology to complex water conditions, simultaneously treating saline water and degrading organic matter. Beyond water treatment, these high-performance materials show great potential in atmospheric carbon and nitrogen cycles.
To better overcome the technical barriers against zero emissions, we established the Carbon Neutrality Innovation Research Center of Shanghai University in 2023.
Our center aims to develop innovative carbon neutrality technologies for carbon capture and catalytic conversion, efficient clean energy development and utilization, green catalytic synthesis and resource efficiency.
Our research is focussed on in-depth understanding of the current state, challenges, and future directions of environmental chemistry. Through academic exchange and collaboration both domestically and internationally, we work with other research teams to address cutting-edge scientific issues and technological innovations in environmental chemistry.
Our ultimate goal is to contribute our knowledge and efforts to global carbon reduction and environmental protection.
You mention atmospheric governance and the nitrogen cycle – could you tell us more about your work in this area?
In the pursuit of zero emissions, it has been demonstrated that actually carbon cycle and nitrogen cycle are inseparable. Our ecological environment is severely impacted by greenhouse gases such as carbon dioxide and atmospheric pollutants like nitrogen oxides, making pollution and carbon reduction an urgent task. The steel industry, a major source of these emissions, faces significant challenges in developing effective denitrification and carbon reduction technologies.
Our team is dedicated to addressing these issues through both fundamental research and technology development for practical applications. In terms of greenhouse gas reduction and high-value utilization, we have established long-term international collaborations with Hokkaido University in Japan and the National Nanotechnology Center at the National Science and Technology Development Agency in Thailand. Our research is focused on the catalytic conversion of major greenhouse gases such as carbon dioxide and methane. By modulating metal-support interactions and alloying active components, we have developed high-performance catalysts for methane dry reforming with carbon dioxide. These catalysts are resistant to sintering and carbon deposition, helping to mitigate the greenhouse effect while achieving high-value conversion of these gases.
In the field of catalytic purification of nitrogen oxide, our team has pioneered the development of low-temperature, poisoning-resistant NOx reduction catalysts. By coupling acid and redox cycles, we have significantly broadened the operational temperature range for NOx reduction. We have also developed a technique for forming high-strength monolithic NOx reduction catalysts (Figure 6), enhancing both mechanical strength and NOx reduction efficiency. Furthermore, we created a low-temperature NOx reduction process tailored for the steel industry's complex flue gas conditions. This process integrates desulfurization, dust removal, and low-temperature NOx reduction, resulting in significant carbon emission reductions.

Our team is also devoted to the high-value conversion of nitrogen oxides. We leverage renewable energy sources such as solar and wind power to drive the photocatalytic and electrocatalytic reduction of atmospheric nitrogen oxides into valuable nitrogen-containing chemicals like ammonia.
This energy-efficient and environmentally friendly technology not only addresses environmental pollution problems but also converts pollutants into reusable ammonia. This approach offers dual benefits for environmental purification and energy conversion.
Through relentless efforts and ongoing research, we aim to further refine and promote photocatalytic and electrocatalytic technologies, significantly contributing to the goal of zero emissions.
Visit our Sustainability Hub to access a suite of on-demand training courses that are free to members, and knowledge resources that will help you embed sustainable principles and practices into everyday work and life.